【Highlights of This Issue】
With the rapid development of tissue clearing technologies, a growing number of biological applications require whole-mount imaging of large-scale transparent samples, which have been successfully applied in fields such as toxicology, neuroscience, and developmental biology. Although light sheet fluorescence microscopy (LSFM) has been widely adopted in this area, optical projection tomography (OPT) remains a classic, cost-effective imaging technique, especially suitable for centimeter-scale samples, such as cleared organs, live embryos, and plants. The basic principle of OPT is similar to that of computed tomography, involving the capture of light intensity distributions by projecting a three-dimensional volume onto a two-dimensional plane from different directions, followed by filtered back-projection to reconstruct the three-dimensional structure. However, traditional methods often suffer from resolution degradation due to the missing cone problem and spatially non-uniform optical aberrations. Additionally, high-resolution imaging typically requires dense axial scanning within a shallow depth of field.
Recently, researchers from Tsinghua University proposed a compact and cost-effective method called the Scanning Light-field Tomography (SLiT) system for high-speed, high-resolution, whole-mount 3D imaging of large-scale samples, achieving near-isotropic resolution. Compared to traditional OPT, which requires hundreds of projections, the reconstruction process for SLiT needs only 24 projections. Additionally, the system eliminates the need for extra axial scanning to increase depth of field, thereby enhancing imaging speed. Building on previous digital adaptive optics technology, Multi-Conjugate Digital Adaptive Optics (MDAO) was introduced to correct complex optical aberrations across multiple angles. The entire 6 × 6 × 6 mm³ volume can be acquired within 30 seconds, achieving a nearly isotropic high-resolution 3D reconstruction (approximately 17 µm for x and z, and 10 µm for y). The versatility and advantages of SLiT have been demonstrated on various samples, such as zebrafish vasculature, cleared mouse brain, vasculature-stained cleared mouse eye, and bone-stained cleared mouse head. This study was published as the cover article in the October 2024 issue of OPTICA.

Fig.1. This study was published as the cover article in the October 2024 issue of OPTICA.
【Technical Background】
Biological imaging technology is a crucial tool in modern life science research, enabling scientists to observe dynamic changes in cells and tissues under physiological and pathological conditions. Traditional biological imaging methods, such as LSFM and OPT, have encountered bottlenecks in imaging speed and precision. LSFM, as a layer-by-layer scanning imaging method, has a natural limit on speed. In pursuit of high resolution, OPT has seen continuous improvements in calibration and reconstruction techniques, incorporating increasingly precise models. However, complex optical aberrations due to heterogeneous samples and variable imaging conditions still negatively impact high-resolution reconstruction and remain unresolved. Additionally, OPT is limited by the depth of field in single-view imaging, often necessitating axial scanning of large-scale samples for high-resolution imaging over extended areas. Currently, no existing OPT system can achieve high-speed, whole-mount imaging of large-volume samples.
As an advanced imaging method, light field technology captures the spatial and angular information of light within a scene, enabling the perception and reconstruction of 3D scenes. Traditional imaging techniques usually only record light intensity on a 2D plane, whereas light field technology further records the direction and angle of light propagation, allowing for multi-view and depth information generation during post-processing. However, due to limitations related to pixel size and microlens dimensions, the spatial resolution of light field microscopy is relatively low. To address this issue, scanning light field microscopy utilizes a moving microlens approach to increase spatial sampling rates, thereby enhancing spatial resolution. Nevertheless, due to sample heterogeneity and complex optical environments, light field technology still faces challenges with view inconsistency and aberration correction. To overcome these challenges, current light field reconstruction algorithms are gradually incorporating more precise optical models and multi-view fusion methods to improve reconstruction accuracy and imaging speed, thus providing more reliable technical support for large-scale 3D imaging and applications such as virtual reality.
【Technical Approach】
The mechanical design of the detection components in the SLiT system is based on a horizontal scanning light field microscope, enabling multi-view data acquisition through a rotatable sample stage (see Figure 2(a)). By simply switching objectives, the resolution and volume size can be adjusted to suit different sample requirements. In the MDAO model, optical aberrations are modeled as 3D distortions caused by multiple virtual phase plates at various depths. By introducing virtual phase plates into a simulated aberration-free optical system, the impact of aberrations on imaging results can be visually demonstrated (see Figure 2(b)). To restore isotropic high resolution through multi-phase plane aberration correction (see Figure 2(d)), the researchers developed a multi-view fusion and deconvolution processing workflow (IMPD). IMPD is based on the phase-space iterative deconvolution method of DAOSLIMIT, incorporating phase-space and back-projection steps (see Figure 2(e)). The phase-space projection step projects the estimated volume onto the light field measurements, while the back-projection step updates the 3D volume based on the error between the measurements and the forward projections. The researchers employed the Alternating Direction Method of Multipliers (ADMM) algorithm for simultaneous iterative updates of the volume and phase.
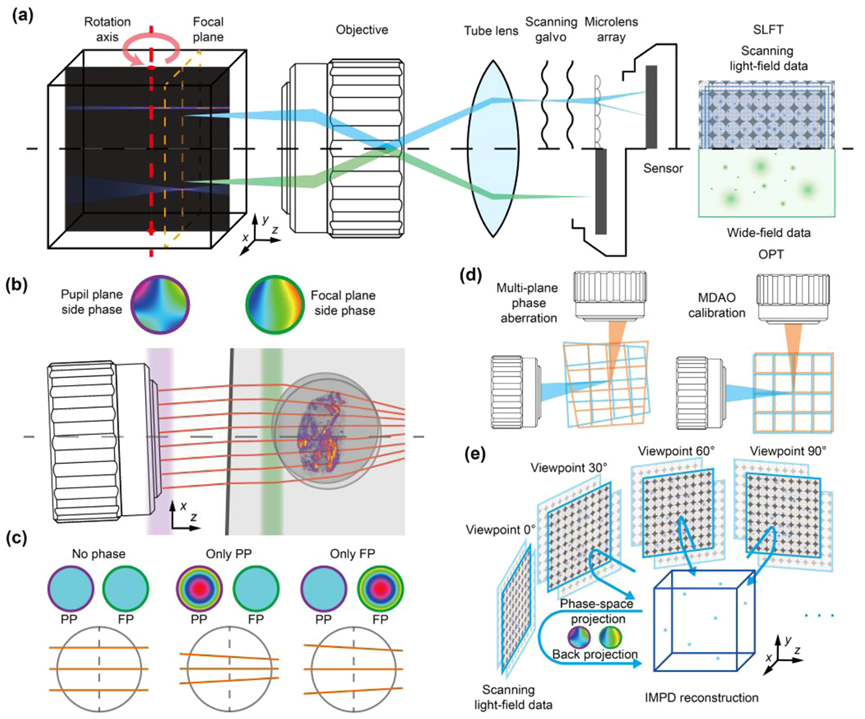
Fig. 2. Principles and performance of SLiT. (a) Comparison of optical system between SLiT and OPT. Compared to the standard wide field detection arm of OPT, the scanning light field setup on the detection arm of SLiT could greatly extend the DOF. The SLiT can cover an extensive volume imaging range during multi-view imaging. (b) Schematic of the multi-layer of virtual phase plates induced by complex imaging environment. When passing non-homogeneous interfaces, light rays are refracted multiple times, causing aberrations in the imaging results. (c) The virtual phase plates conjugated on different planes cause different aberrations. When both PP and FP have a constant phase, parallel light rays remain parallel as they propagate to the focal plane. When the PP has a focusing phase, the parallel light rays will tilt as they reach the focal plane, causing a significant shift at their intersection with the focal plane. When the FP has a focusing phase, the parallel light rays will also tilt as they reach the focal plane, but the shift at their intersection with the focal plane is minimal. This example demonstrates that the shift and tilt of light rays caused by a multilayered inhomogeneous medium cannot be represented by the phase within only one plane. (d) Aberrations from each viewpoint caused information mismatches between viewpoints, which disrupted the reconstruction. By applying MDAO, all aberrations are corrected, maximizing the recovery of reconstruction quality. (e) Schematic of the IMPD reconstruction algorithm. First, the estimated volume undergoes phase-space projection. During this process, the MDAO method is used to guide the projection process in order to correct for aberrations. By comparing the phase-space domain images estimated through projection with the measurements, the estimated error can be determined. This error is then used to update the volume through the back-projection process with guidance from the MDAO method. Finally, the high-reoslution reconstruction volume can be obtained through repeated iterations from multiple different angles.
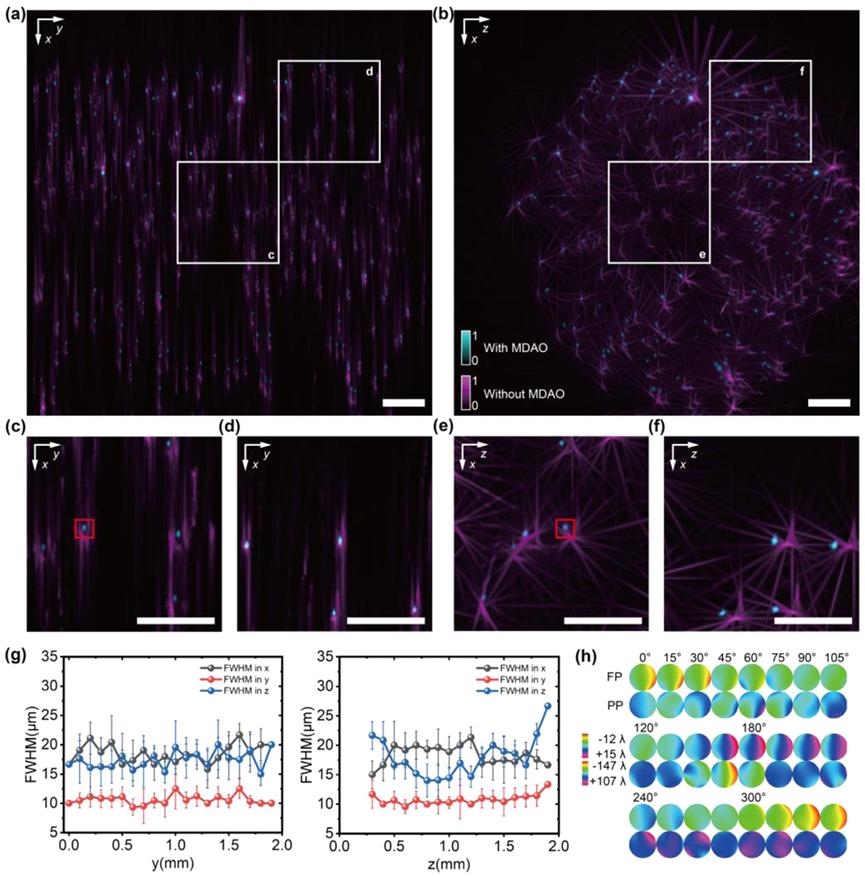
Fig. 3. MDAO enhanced the reconstruction performance SLiT imaging result of fluorescent beads and their respective resolution (FWHM) (a, b) MIPs along the z-axis and y-axis of reconstructed fluorescent beads with a 2×/0.055NA objective by SLiT (with MDAO correction in cyan and without MDAO correction in magenta). SLiT improved the resolution along the x, y, and z axes across the entire volume. (c, d, e, f) Zoomed MIP images from the reconstructed volume of (a) and (b) marked by white boxes, the selected two region sizes from (a) are both 0.5mm×0.5mm ×0.5mm. (g) the lateral and axial average point FWHM for SLiT (with MDAO correction) along the y-axis and z-axis in the coordinate system of (a). The resolution across the entire volume is nearly uniform. Due to the aberration, images taken from different viewing angles have limited registration accuracy in the x and z directions, which leads to the resolution in the x and z directions (around 17 µm) being worse than the resolution in the y direction (around 10 µm). (h) The phases of MDAO from 24 viewpoints. PP stands for pupil-plane, and FP stands for focal-plane. Scale bars in (a) and (b) are 200 µm.
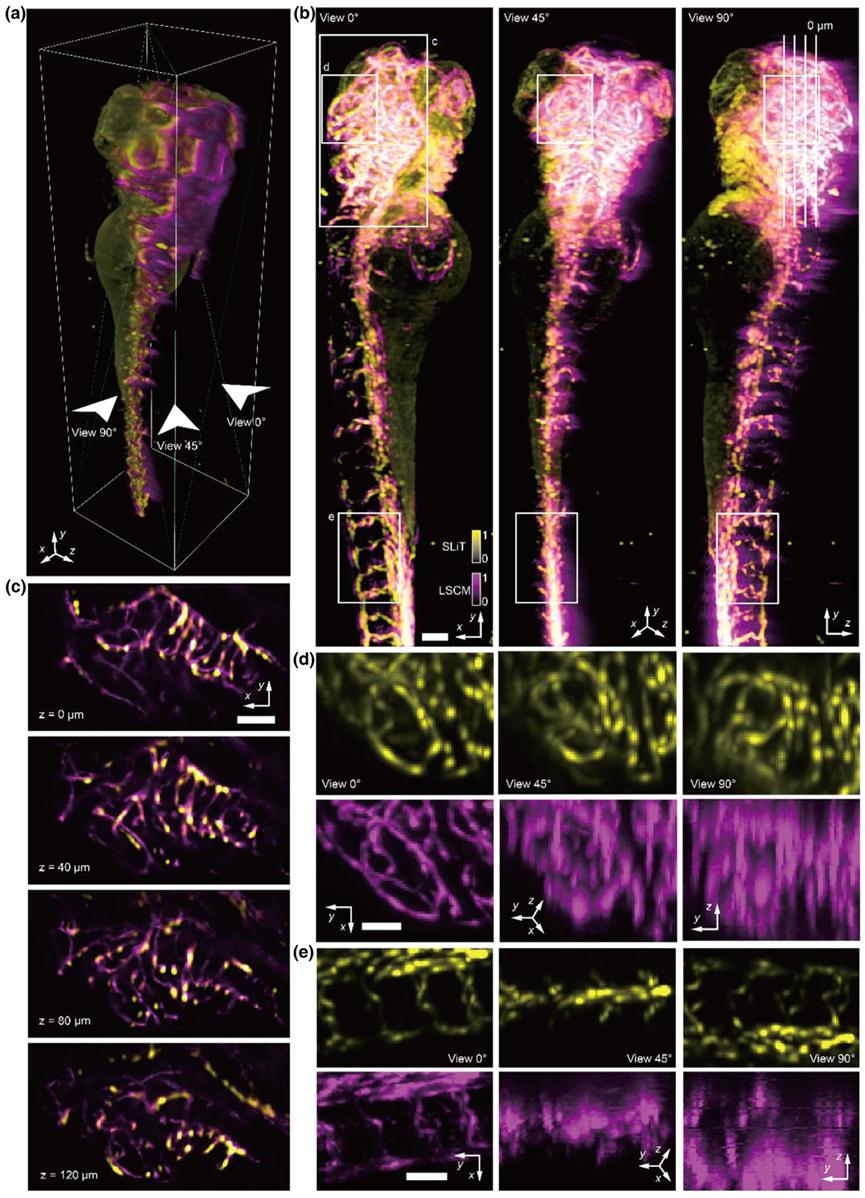
Fig. 4. Comparisons of the results on zebrafish vessels imaged by SLiT and LSCM (a) 3D rendered volume of multimodal zebrafish imaging results, with rotation axis marked on the side. SLiT result was shown in yellow, and the LSCM result was shown in Magenta. LSCM data was captured from view 0. (b) MIPs of multimodal zebrafish imaging results, which projected from view 0, view 45, and view 90. (c) slice stack of multimodal zebrafish imaging results. The stack is sliced Perpendicular to the view 0. The position of the stack was marked in (a) with white boxes. (d, e) MIP of subsection volume of zebrafish imaging results. Results from SLiT and LSCM were separated into yellow and Magenta channels. The position of the volume was marked on (a) with green and blue boxes. Scale bars in (b, c) are 100 µm, and in (d, e) is 50 µm.
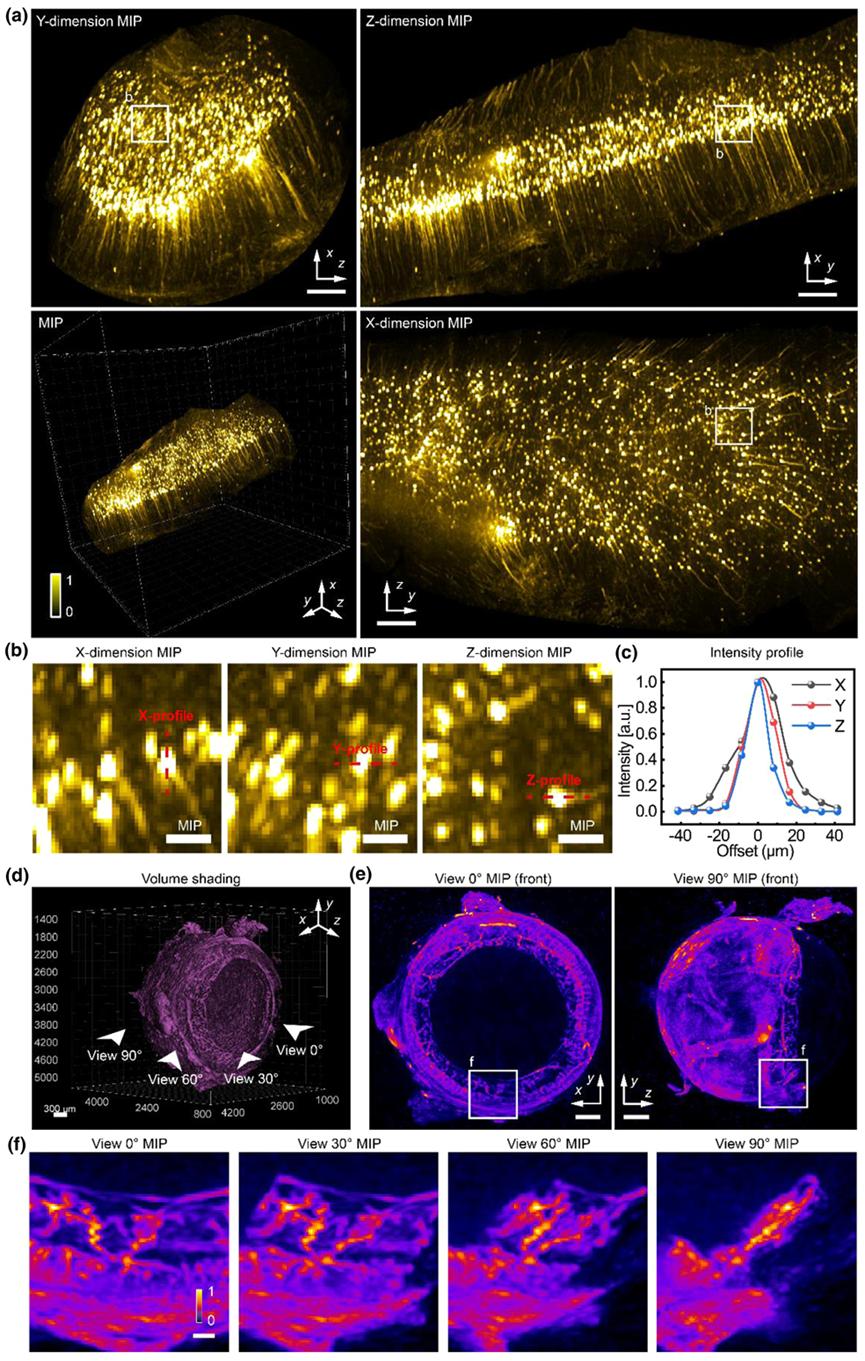
Fig. 5. Mesoscale volumetric imaging of diverse clear tissue (a) MIPs of reconstructed cleared mice brain layer-4 cortex with a 2×/0.055NA objective by SLiT with 24 views and a single view. The bottom right image is reconstructed cleared mice brain layer-4 cortex rendered in 3D.(b) the sub-volume MIP of the neuron, projected from x, y, and z dimensions. (c) The intensity profile of the neuron corresponds to red lines in b. (d) The volume shading results from the blood vessels in cleared mice eyes. (e) The MIP of the eye is projected from the x and z dimensions. Only half of the eye is included in the MIP to show the delicate details. (f) the sub-volume MIP of the iris blood vessels. The volume corresponds to the indicated view in d. Scale bars in (a) are 500 µm, in (b) are 100 µm, in (d) are 300 µm, in (e) are 500 µm, in (f) are 100µm.
To evaluate the performance of SLiT, the researchers performed 3D imaging of fluorescent microspheres with a diameter of 1 µm, randomly dispersed in 1% agarose gel, using an objective lens with a 0.055 NA. Without MDAO correction, there was significant misalignment between multiple views, resulting in obvious artifacts and lower resolution. After MDAO correction, the multi-view data were properly aligned, yielding higher spatial resolution without artifacts (Figures 3(a)–3(f)). Additionally, by calculating the full width at half maximum (FWHM) of the microspheres in the SLiT reconstruction, it was found that the lateral and axial resolutions in the experiment were nearly consistent (approximately 17 µm in the x and z directions and 10 µm in the y direction), and remained stable throughout the entire volume after multi-position 3D aberration correction. The estimated phases from different views are shown in Figure 3(h). The alignment between views and the correlation with the reconstruction results validated the effectiveness and reliability of the MDAO estimates.
To further assess SLiT's performance on biological samples, the researchers performed large-volume imaging of developing zebrafish vasculature using a 5×/0.14 NA objective lens. As a reference, the same sample was also imaged using a laser scanning confocal microscope (LSCM) with a 4×/0.13 NA objective lens for comparison. While the LSCM results confirmed the accuracy of SLiT, LSCM failed to image the zebrafish’s eye on the posterior side due to scattering effects from the sample. SLiT achieved higher axial resolution at a faster imaging speed than LSCM, facilitated by its rotational process. Maximum intensity projections (MIPs) from various views showed consistent details of the vasculature structure (Figure 4(b)). Some mismatches were possibly due to aberrations in LSCM and the zebrafish’s growth during sample storage. In view 90, the LSCM image faded on the zebrafish’s posterior side due to scattering by anterior tissue. The generated slice stacks displayed vascular structure details and tomography capabilities (Figure 4(c)). Several sub-volumes were further magnified to show the resolution improvement of SLiT (Figures 4(d) and 4(e)). At view 0, SLiT (in yellow) was largely consistent with the LSCM vascular structure. However, at views 45 and 90, the LSCM structure became blurred due to lower axial resolution, while SLiT continued to display clear structure.
To further demonstrate SLiT’s advantages for large-scale cleared tissue, the researchers imaged several different sample types across multiple scales, including neurons in a cleared mouse brain (Figure 5), vasculature in a cleared mouse eye (Figure 5), and the cleared, bone-stained mouse head.