The ability to observe dynamic microenvironments within living animals at high speed, high resolution, and over long durations has run through the centuries-long development of microscopy, serving as an icebreaker for life sciences and medical research, and as a pioneer in discovering new phenomena and revealing new mechanisms. However, due to limitations such as the 3D distribution of tissues, optical aberrations, phototoxicity, and other tenacious issues, the challenge of conducting high-speed, subcellular resolution observations over long periods in the living environment of mammals has remained unresolved, greatly constraining in-depth researches in brain sciences, oncology, and immunology.
On May 25, 2021, a research paper titled “Iterative tomography with digital adaptive optics permits hour-long intravital observation of 3D subcellular dynamics at millisecond scale,” was published in Cell, completed by the research teams led by Prof. Qionghai Dai from the Institute of Brain and Cognitive Sciences and the Department of Automation, and Li Yu from the School of Life Sciences at Tsinghua University. The team uniquely proposed a digital adaptive optics framework, invented the scanning light field imaging technology. After three years of efforts, they created a Digital Adaptive Optics Scanning Lightfield Mutual Iterative Tomography (DAOSLIMIT). DAOSLIMIT achieves a diffraction-limited resolution of 220 nm laterally and 400 nm axially within an imaging field of view of 225×225×16 μm³. It extends the continuous 3D observation duration of living animals from minutes to hours at the millisecond scale, realizing an improvement of two orders of magnitude in the spatiotemporal resolution of in vivo imaging and a reduction of three orders of magnitude in phototoxicity. DAOSLMIT provides a new tool for revealing the interactions between multiple cells and organelles in living mammals.
Traditional light field microscopes are capable of recording multi-angle information of a volumetric sample in a single shot, enabling rapid 3D imaging. However, they cannot simultaneously achieve high spatial and angular resolution. DAOSLIMIT utilizes the slight vibrations of a high-speed 2D galvanometer to introduce spatial constraints, obtaining high-resolution 4D light field information in space and angle with full photon efficiency. This enables incoherent aperture synthesis, and by leveraging the spatiotemporal continuity priors of the sample, it avoids the loss of temporal resolution caused by scanning.
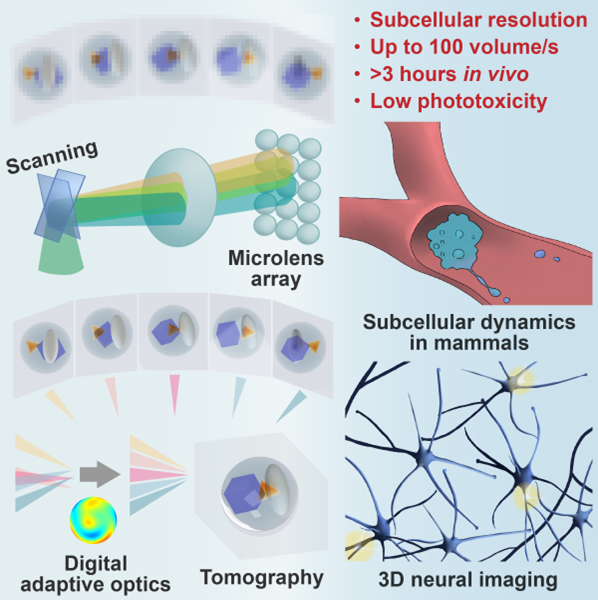
Figure1 | Schematic of System Concept and Application
One major bottleneck constraining the observation of living tissue is the severe optical aberration caused by the 3D spatial distribution of the sample refractive index, which significantly reduces the spatial resolution of microscopic imaging. Traditional adaptive optics microscopes, derived from astronomical observation, can only correct aberrations in a small field-of-view area, even with complex hardware and software. DAOSLIMIT establishes a new framework for digital adaptive optics (DAO) imaging, which, without additional wavefront sensors or spatial light modulators, decouples signal acquisition from adaptive wavefront correction by obtaining 4D full light field information. It achieves large-scale multi-site adaptive optical correction in the post-processing stage, enhancing the spatial resolution to the optical diffraction limit.
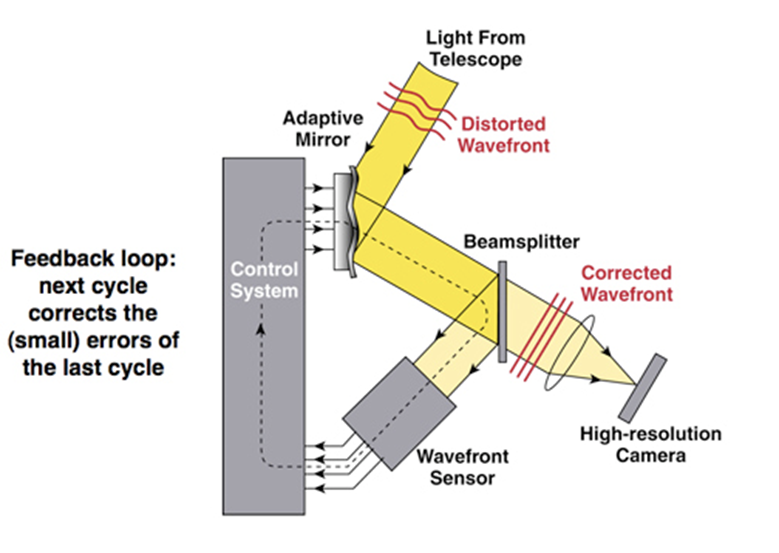 Figure2 | Schematic of Adaptive Optics Principles
Figure2 | Schematic of Adaptive Optics Principles
The issue of phototoxicity caused by illumination is another long-standing challenge in intravital fluorescence imaging. Spinning disk confocal microscopy (SDCM) and two-photon microscopy have low photon efficiency, and continuous high-speed imaging for just a few minutes could lead to severe photobleaching and photodamage on the sample. Light sheet microscopy mitigates this problem by only exciting the focused region, but it cannot maintain subcellular resolution in opaque tissues. DAOSLIMIT adopts a simultaneous excitation and collection mode within the same 3D region of interest. By expanding the axial depth of field, it makes full use of all emitted photons for imaging. Compared to conventional microscopes, DAOSLIMIT requires only mW-level excitation intensity to achieve sufficient SNR, thereby reducing phototoxicity by three orders of magnitude. It is the first time internationally that millisecond-scale high-speed continuous observation for several hours within live mammalian organisms has been achieved.
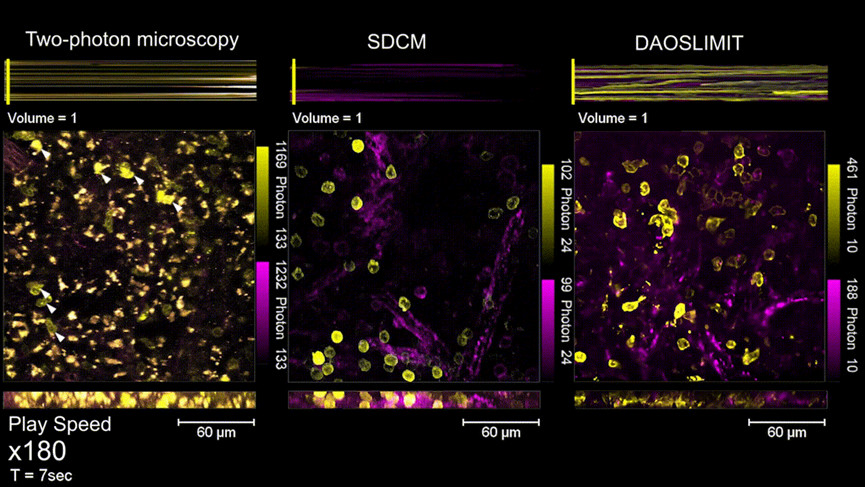
Figure3 | In vivo imaging experiment of mouse spleen, with a comparison of phototoxicity between DAOSLIMIT and SDCM, Two-photon microscopy
The migrasome, recently discovered and named by Professor Li Yu’s laboratory, is a newly discovered organelle that plays important roles in embryonic development and the maintenance of immune system. With the help of DAOSLIMIT, a new field of research into the functions of migrasomes in the living mammals has been opened up. Researchers labelled neutrophils and blood vessels separately and performed multicolor imaging within the living mouse livers, observing for the first time the formation and changes of migrasomes and retraction fibers in the mammals. “With DAOSLIMIT, we observed that immune cells leave many migrasomes behind as they move in the blood vessels of the mouse liver. Immune cells may achieve widespread communication and long-distance interactions between cells by producing migrasomes, which could be a new mechanism. These migrasomes are like the beacon towers of the Great Wall, potentially playing a key role in signaling during a series of complex reactions, such as immune surveillance,” Professor Li Yu said.
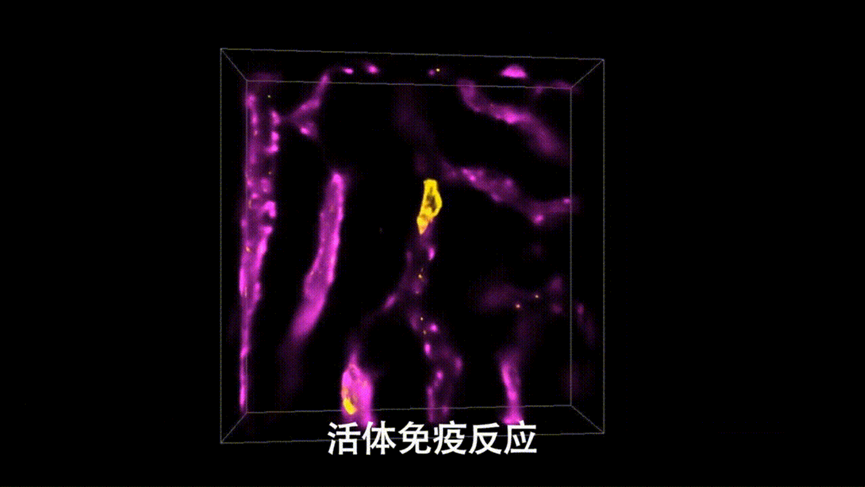
Figure4 | High-speed, High-resolution Imaging of In Vivo Immune Response in Mouse Livers
Researchers further injected human tumor cells into the zebrafish larvae and, with the extremely low phototoxicity of DAOSLIMIT, discovered new phenomena of tumor cells actively adapting to the environment through vesicles and retraction fibers during continuous, high-speed and long-term observations. Professor Dai pointed out, “The new technology provides a new tool for life sciences and medical research. These interesting new phenomena are just the tip of the iceberg. With technological advancements, the discoveries of more new phenomena and the revelation of new mechanisms are expected in the future to aid significant breakthroughs in basic research fields, such as brain sciences, oncology, and immunology.”
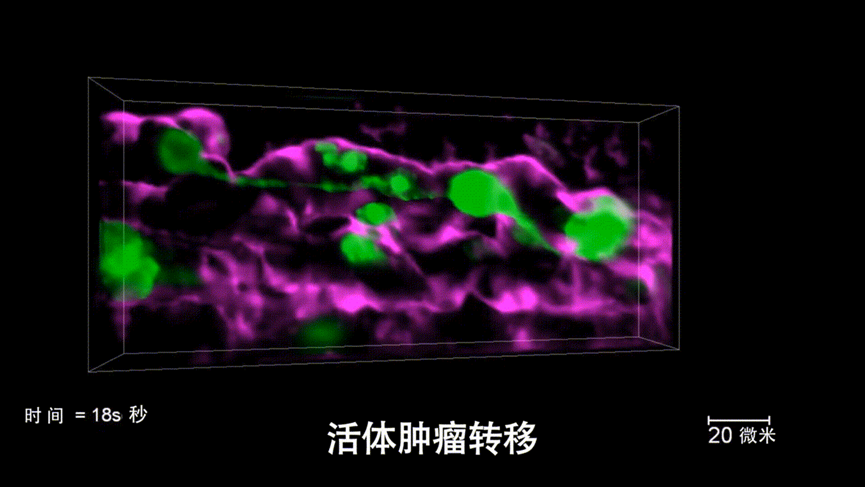
Figure5 | High-speed, high-resolution imaging of live tumor metastasis in live zebrafish
The postdoctoral researcher Wu Jiamin, doctoral student Lu Zhi from the Department of Automation, and postdoctoral researcher Jiang Dong from the School of Life Sciences are the co-first authors of the paper. Professors Dai from the Department of Automation, Beijing National Research Center for Information Science and Technology, and Institute of Brain and Cognitive Sciences, Associate Researcher Fan Jingtao, and Professor Yu from the School of Life Sciences are the co-corresponding authors of the paper. This work was also supported by the National Natural Science Foundation of China and the Ministry of Science and Technology of China. The related technology is being commercialized.
References
[1] Wu, J. et al. Iterative tomography with digital adaptive optics permits hour-long intravital observation of 3D subcellular dynamics at millisecond scale. Cell. (2021).
[2] Max C. "Introduction to adaptive optics and its history." American Astronomical Society 197th Meeting. NSF Center for Adaptive Optics University of California at Santa Cruz and DOE Lawrence Livermore National Laboratory. (2001).