Sepsis caused by bloodstream infections is one of clinical practice's most severe infectious diseases. Capsulated bacteria are among the most common pathogens responsible for bloodstream infections, including Gram-positive Streptococcus pneumoniae and Staphylococcus aureus, as well as Gram-negative Klebsiella pneumoniae and Escherichia coli. The interaction between pathogens and the host immune system plays a decisive role in the progression and outcome of bloodstream infections. The spleen, the largest secondary lymphoid organ in the human body, is rich in various innate immune cells and lymphocytes. Clinical studies have shown that individuals with splenic dysfunction are particularly susceptible to bloodstream infections caused by capsulated bacteria, with a mortality rate as high as 50–70%. However, the cellular and molecular mechanisms by which the spleen exerts its anti-infective function remain unclear.
On March 12, 2025, Professor Jingren Zhang from the School of Basic Medical Sciences at Tsinghua University, Academician Qionghai Dai from the Department of Automation at Tsinghua University, and Assistant Professor Haoran An from the Institute of Medical Technology at Peking University Health Science Center collaborated to elucidate the critical immune function of splenic red pulp macrophages (RPMs) in clearing Streptococcus pneumoniae from the bloodstream. They clarified the cooperative mechanisms among different tissue-resident macrophages (TRMs) in anti-infective immunity. This study, titled "Splenic red pulp macrophages eliminate the liver-resistant Streptococcus pneumoniae from the blood circulation of mice", has been published in Science Advances.
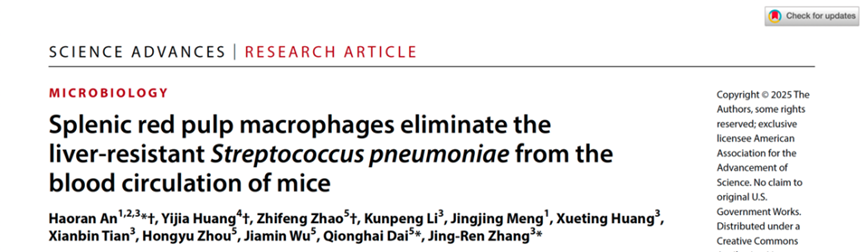
The study employed two strains of Streptococcus pneumoniae with low virulence (LV) and high virulence (HV) to establish a mouse bloodstream infection model. Using pharmacological treatments and gene knockout mouse models, the team demonstrated that splenic RPMs are the key immune cells controlling HV Streptococcus pneumoniae infections, while neutrophils play a secondary role. To directly visualize the interactions between immune cells and pathogenic bacteria in the spleen, the researchers employed their self-developed two-photon synthetic aperture microscopy (2pSAM) imaging technology for real-time in vivo monitoring over four hours. The results showed that bacteria in the bloodstream rapidly entered the spleen, where the majority were captured by RPMs in the red pulp region, with a smaller portion being phagocytosed by neutrophils (Figure 1).
Figure 1. Splenic RPMs as the key immune cells recognizing high-virulence Streptococcus pneumoniae.
A: Representative in vivo microscopy field of view depicting interactions between splenic immune cells and pathogens.
B and C: Dynamic changes in bacterial capture by the spleen under conditions of PRM and neutrophil depletion.
How do splenic RPMs recognize Streptococcus pneumoniae? Further research revealed that natural antibodies in the host serum are essential effector molecules in the clearance of HV bacterial strains by the spleen. Through specific competitive assays, the team identified that natural antibodies target the phosphocholine (PC) antigen on the surface of Streptococcus pneumoniae. Using a recombinant monoclonal PC antibody (T15 IgM), they confirmed this crucial function (Figure 2). Additionally, they found that the complement system in the serum serves as a key downstream effector pathway of natural antibodies, unveiling the cooperative role of these two major innate immune factors in bacterial defense.

Figure 2. Phosphocholine natural antibodies enhance splenic RPM clearance of high-virulence Streptococcus pneumoniae: following the injection of 5×106 CFU native bacteria and 25 μg T15 IgM-opsonized bacteria in Cd19-/- mice, 2pSAM was used to monitor pneumococcal capture.
This study also uncovered how HV bacteria evaded Kupffer cell (KC) immune surveillance are ultimately cleared. The findings indicate that in KC-deficient models (such as those with severe liver disease), the immune clearance of LV bacteria similarly depends on splenic RPMs. This suggests that during systemic infections, tissue-resident macrophages from different host organs perform specialized functions to collectively maintain immune defense (Figure 3).
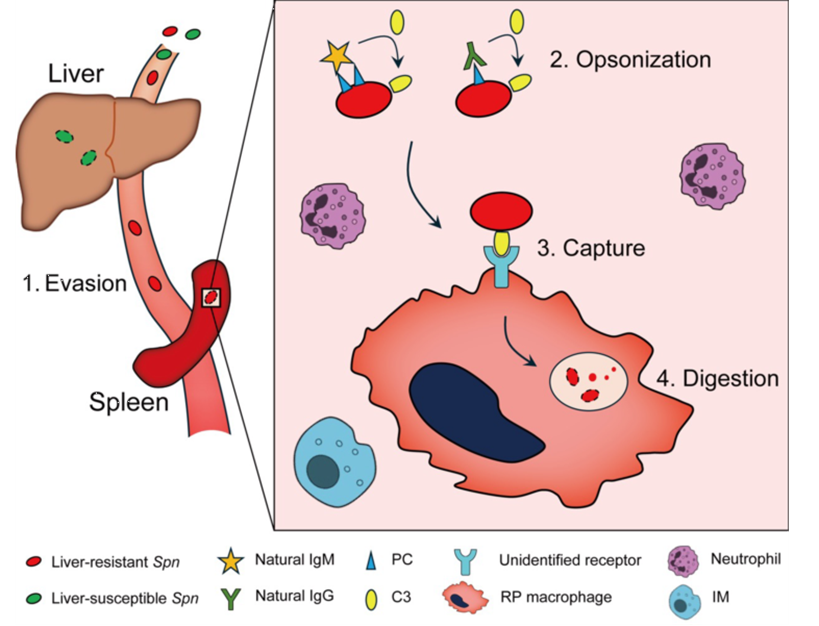
Figure 3. Schematic diagram illustrating the mechanism by which splenic RPMs utilize natural antibodies and complement to clear bloodstream pathogens.
In summary, this study reveals the immune mechanism by which splenic RPMs rely on natural antibodies and complement to eliminate highly invasive Streptococcus pneumoniae, providing an explanation for the increased susceptibility of individuals with splenic defects or splenectomy to bacterial infections. These findings offer new scientific insights into the immune function of the host in defending against bacterial infections.
Haoran An from the Institute of Medical Technology at Peking University Health Science Center, Yijia Huang from the School of Basic Medical Sciences at Tsinghua University (now affiliated with Wenzhou Medical University), and Zhifeng Zhao, a postdoctoral researcher in this laboratory from the Department of Automation at Tsinghua University, are the co-first authors of this paper. This research was supported by the National Key R&D Program, the National Natural Science Foundation of China, the "Sailing Program" of Peking University Health Science Center, and the Beijing Advanced Innovation Center for Medical Sciences.