The significant differences between in vitro and in vivo environments can markedly affect the function and phenotype of cells. The interactions within different types of cells under physiological and pathological conditions are difficult to be reproduced in vitro. Thus the long-term observation of the transient processes at cellular and even subcellular level in living organisms is the key to unlocking the black box of life and revealing the mechanisms underlying human health and disease. The spinning disk confocal microscopy (SDCM) and two-photon microscopy, the commonly used microscopes reject the out-of-focus fluorescence with a pinhole or multiphoton excitation and only capture the in-focus photons within a very shallow depth of field, leading to high signal-to-background ratio (SBR). However, these approaches inevitably reduce 3D imaging speed, and result in strong phototoxicity due to repeated excitation of out-of-focus layers during 3D imaging in vivo. The scanning light-field microscopy (sLFM) [1], developed by Tsinghua University in 2021, is able to achieve 3D subcellular imaging at an ultra-high speed equivalent to camera frame rates, while reducing phototoxicity by three orders of magnitude, has been widely used in brain science, oncology and immunology researches. But strong background fluorescence within complex diverse intravital environments still causes substantial degradation of imaging fidelity.
On May 27, 2024, Nature Biotechnology published a research article entitled “Long-term intravital subcellular imaging with confocal scanning light-field microscopy”[2], which was accomplished by Tsinghua University and Hehu Technology. To break through the trade-off between background fluorescence and imaging fidelity, the research team developed a new confocal scanning light field microscope (csLFM) through nearly three years of efforts. csLFM achieves aberration-corrected high-speed 3D subcellular imaging with both optical sectioning and low phototoxicity by developing a line-confocal scheme upon sLFM and a new 3D reconstruction algorithm and has gained an all-around performance upgrade compared with SDCM: its 3D imaging speed significantly increased by at least 20 times, while the phototoxicity is reduced by 130 times. In the foreseeable future, csLFM is set to replace traditional confocal microscopes, can serve as a unprecedented tool to enable the studies of the interaction between cells and subcellular structures with higher fidelity in deep tissue or densely labeled fluorescent samples, which will provide a solid technical support for analyzing complex mechanisms in brain science, immunology, pathology, etc.
Fig. 1 | Paper page
Based upon the advantages of sLFM in high spatiotemporal resolution and long-term observation, the research team further improved its imaging quality and practicability of diverse life science and medical applications by realizing the function of optical sectioning. As shown in Fig. 2, with synchronization of an axially elongated line-confocal illumination and the camera rolling shutter of multiple rows, csLFM can selectively collect fluorescent signals from in-focus volume within a compact system.
Owing to the inherent 3D perception capability of sLFM, there is no need for very narrow slits or pinholes to eliminate background fluorescence. The axially elongated line-confocal illumination allows for the specific removal of background fluorescence outside the effective imaging range, without sacrificing three-dimensional light efficiency and imaging speed. csLFM achieves 12 dB improvement in SBR and equips with optical sectioning and digital adaptive optics for high-fidelity imaging of densely labelled samples while maintaining the same effective axial coverage, near-diffraction-limit resolution in complicated environment, which supporting precisely capturing organelles and other subcellular structures with high fidelity.
Fig. 2 | Principle of csLFM
The spleen is a prominent organ of the immune system, containing a large number of immune cells executing a variety of immune reactions. However, intravital imaging of the spleen at subcellular level is challenging due to its dense tissue density, strong background fluorescence, and sensitivity to photodamage. With the excellent optical sectioning capability of csLFM in rejecting background contamination and extremely low phototoxicity, the researcher team has successfully achieved the continuous and high-speed, high-resolution 3D imaging of the spleen immune microenvironment in vivo for over several hours, and comprehensively characterized how the immune system works together in its native state. The researchers unexpectedly observed that NK cells can adhere to macrophages by generating retraction fibers and directionally produce migrasomes inside the macrophages. The phenomenon of migrasome delivery suggests a potential signaling synergistic mechanism to enhance innate adaptive immune surveillance. It is noteworthy that after effectively eliminating Poisson-dominated noises originated from the background intensity, csLFM is able to finely resolve tiny organelle structures. For example, the formation process of retractosomes, which are only 50-250 nm in size, is clearly and dynamically rendered by csLFM in vivo in mice.
Fig. 3 | High-speed, high-resolution, high-fidelity imaging of the delivery of migrasomes in living mouse spleens
Fig. 4 | High-speed, 3D imaging of retractosome formation in living mouse livers
Orientation selectivity plays a key role in the process of visual neural coding. The researchers recorded the calcium dynamics in primary visual cortex under multiple rounds of stimuli with a moving grating by using csLFM. In contrast to sLFM results, in which many weak responses were flooded, csLFM greatly reduced the signal crosstalk caused by tissue scattering and dense fluorescence labelling. Quantitative results showed that the orientation selectivity index (OSI) obtained by csLFM reached 52% within a depth of 200-300 μm in the cortex. csLFM rivals the imaging performance of two-photon microscopy with higher spatial resolution and is expected to be widely used in brain science research to study the dynamic interaction process between neurons and different types of cells.
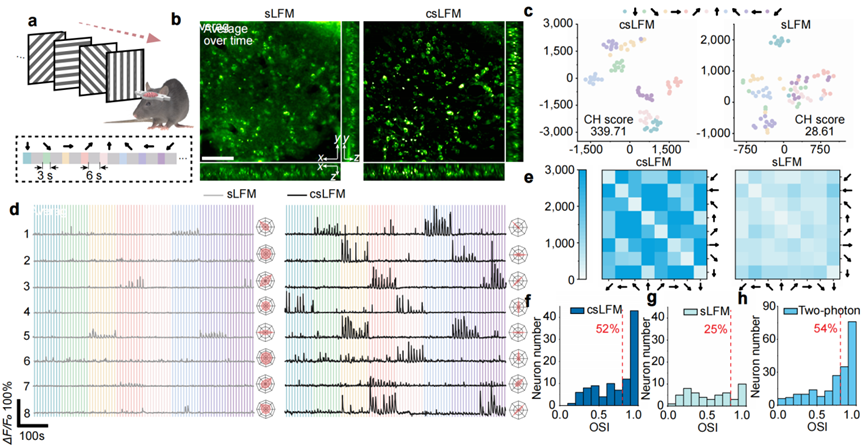
Fig. 5 | csLFM improves fidelity and neuronal orientation selectivity in high-speed 3D neural imaging in awake mice.
Transmembrane voltage indicator which measures the real response of single neuron at a millisecond scale, provides a new non-invasive tool to record large-scale neural activity in living animals. However, the higher speed response also poses a significant challenge to microscopy. csLFM achieved the large-scale high-fidelity signal recording of almost all dopaminergic neurons in the brain of an awake behaving Drosophila at a depth of 100 μm and speed of 150 VPS. Compared to sLFM, csLFM obtained nearly a threefold increase in identified spike number and 1.5 times enhancement in spike amplitude quantitatively. Moreover, csLFM reduced the background and improve the image contrast. This method will greatly facilitate the application of voltage indicators in neuroscience, enables insightful understandings of the function and mechanism of neural circuits, and has great significance for the diagnosis and treatment of related diseases caused by abnormal neural circuit development.
Fig. 6 | csLFM can be applied in voltage imaging with better fidelity.
It is reported that this technology has been transformed into a microscopy product SLiM2000 in Hehu Technology Co., LTD. To bridge the gap between academic research and commercialized products, and promote the application of this advanced scientific instrument, the product development and academic research of csLFM proceeded simultaneously in the past three years, and SLiM2000 product was officially released at the same time with the academic paper recently (http://www.hehutek.com/cn/product/slim2000). The product supports high-fidelity 3D imaging with subcellular resolution of up to 150 volumes per second while maintaining the same resolution and optical sectioning as SDCM. , increasing the 3D imaging speed by more than 100 times. In addition, SLiM2000 equipped with a motorized stage and a customized sample adapter, can achieve autofocus, AF tracking and real-time rendering. Moreover, with the integration of pre-developed denoising algorithm and artificial intelligence analysis, SLiM2000 is able to obtain more in-depth data analysis. SLiM2000 allows scientists to further expolre high-speed neuroimaging, observation of the immune microenvironment, and other large-scale studies of cell-cell interactions in vivo. For more information, please consult Hehu Technology via emails (sales@hehutek.com).

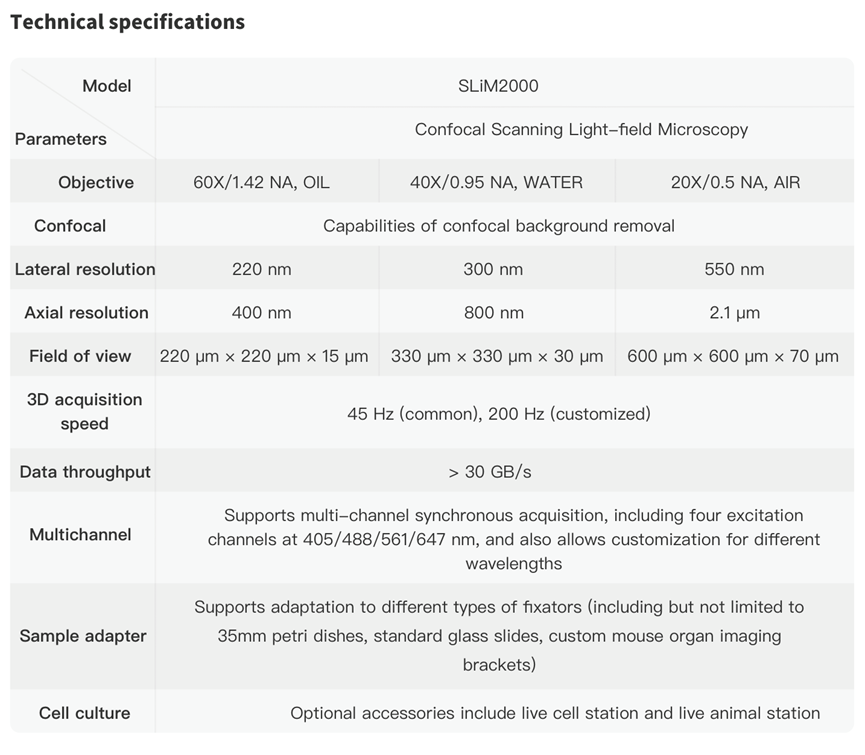
Fig.7 | SLiM2000 product and specifications
Dr. Lu Zhi, Mr. Zuo Siqing and Mr. Shi Minghui from Tsinghua University are co-first authors of this research. Professor Dai Qionghai, Associate Professor Wu Jiamin and Professor Yu Li from Tsinghua University are co-corresponding authors. The research was supported by National Natural Science Foundation of China and China postdoctoral science foundation.
Original link: https://doi.org/10.1038/s41587-024-02249-5
References:
[1] Wu, J. et al. Iterative tomography with digital adaptive optics permits hour-long intravital observation of 3D subcellular dynamics at millisecond scale. Cell 184, 3318-3332 (2021).
[2 ]Lu, Z. et al. Long-term intravital subcellular imaging with confocal scanning light-field microscopy. Nature Biotechnology (2024). https://doi.org/10.1038/s41587-024-02249-5